New Zealand venture capital firm GD1 (Global from Day One) has led a $US18 million ($NZ29.9 million) second-tranche Series A funding round for med-tech company Alimentary.
The over-subscribed round was supported by the American Gastroenterological Association’s GI Opportunity Fund, Japan’s Olympus Innovation Ventures, New Zealand’s Icehouse Ventures and Australian superannuation fund Hostplus. Existing investors also contributed follow-on funding.
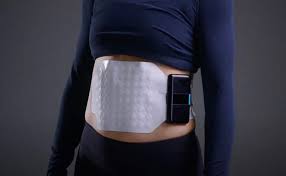
Auckland-based Alimetry has developed a wearable medical device that helps diagnose gastric diseases by measuring the pattern and intensity of electrical waves in the stomach. The flexible device, which contains an array of sensors, is pasted across the abdomen and measures electrical signals as food is digested. The signals are interpreted by the system’s app, trained on a large history of cases. This helps clinicians diagnose more accurately in an area of medicine where treatment has largely had to rely on trial and error.
Alimetry decided to raise additional funding as increasing numbers of US hospitals began using its technology following receipt of the company’s fourth US Food and Drug Administration (FDA) clearance, and after the completion of more than 30 clinical studies.
In 2022, New Zealand venture capital firm Movac led an over-subscribed $NZ16 million Series A investment round for Alimetry. In total, the company has raised just under $NZ50 million.
The potential of diagnosing stomach issues from electrical signals had been recognised for decades but had not been followed up because the signals are weak – 100 times weaker than signals from the heart. Advances in sensor technology and artificial intelligence (AI) encouraged Alimetry’s founders Dr Greg O’Grady, a professor in gastroenterology at the University of Auckland Medical School, and Armen Gharibans, a bio-engineer from the University of California, San Diego, to work on interpreting gastric electrical signals.
Their technology was developed at the Auckland Bioengineering Institute and Alimetry was formed in 2019.
“Alimetry was designed to introduce clarity into a field that has involved lengthy, uncertain diagnostic journeys,” O’Grady said. “It gives clinicians the tools they need to quickly, and correctly, diagnose patients so that we can move on from trial and error − and guesswork − into clarity of care and personalised medicine.”
O’Grady said the funding environment had changed markedly since Alimetry had first sought Series A funding in 2021. Investors had become much more cautions and he had been told due diligence was generally taking longer. Despite that, he was pleased with the outcome of Alimetry’s new fundraising, particularly with the investors who had committed.
Involvement of the American Gastroenterological Association represented validation of the technology from its users while involvement of Olympus showed a major player in medical imaging wanted to strategically align with Alimetry.
O’Grady said Olympus was a strong player in medical imaging diagnostics of the gut.
GD1 co-managing partner Vignesh Kumar said the calibre of the new supporting investors backed up his firm’s view that Alimetry had made steady progress both with its technology and regulatory clearance path.
He said the new funding should give the company a three-year runway to ramp up selling its hardware in its main market, the US.
Much of the early R&D funding which developed the Alimetry device came from non-dilutive sources with over $NZ5 million provided by the NZ government and additional funding from the US National Institute of Health.
This had enabled the company to make progress with its technology development while the founders retained most of the equity.

The founders did, however, accept their first external funding, in late 2019, from IP Group. Another early investor was New Zealand deep-tech specialist venture capital firm Matū. The University of Auckland’s UniServices retains a modest stake in the company resulting from the spin-out of the IP.
Image: The Alimetry device.
